All Categories
Featured
Table of Contents
Buy A0580 - Sigma-aldrich in TEXAS
Publisher Copyright: extcopyright 2021, The Author(s), under exclusive licence to Springer Nature Switzerland AG. Copyright: Copyright 2021 Elsevier B.V., All rights reserved.", year = "2021", month = oct, doi = "10. 1007/s40266-021-00882-2", language = "English", volume = "38", pages = "887--910", journal = "Drugs and Aging", issn = "1170-229X", publisher = "Adis International Ltd", number = "10",TY - JOURT1 - Safety and Tolerability of Natural and Synthetic Cannabinoids in Older Adults, T2 - A Systematic Review and Meta-Analysis of Open-Label Trials and Observational Studies, AU - Pisani, Sara, AU - Mc, Goohan, Katie, AU - Velayudhan, Latha, AU - Bhattacharyya, Sagnik, N1 - Funding Information: SB and LV received funding from Parkinson’s UK (grant number G-1901).

16/126/53]. The authors acknowledge support from the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. No other disclosures were reported.
Copyright: Copyright 2021 Elsevier B.V., All rights reserved. PY - 2021/10Y1 - 2021/10N2 - BACKGROUND AND OBJECTIVE: Although cannabinoid-based medications are increasingly used by older adults, their safety and tolerability in this age group remain unclear. The purpose of this systematic review was to examine the safety and tolerability of cannabinoid-based medications by conducting a meta-analysis of open-label observational studies of cannabinoid-based medications for all indications in individuals with a mean age of ≥50 years.
Cannabis And Parkinson's Disease: Weeding Out The Details now available in NC - limited time only
Study quality was assessed using an adapted version of the Grading of Recommendations Assessment, Development and Evaluation criteria and Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines were followed. We included studies that (a) were published from 1990 onwards; (b) included older adults (mean age ≥50 years); and (c) provided data on the safety and tolerability of medical cannabinoids.
Risk of adverse events, serious adverse events and withdrawals was computed as the incidence rate (IR). Separate analyses were conducted by the cannabinoid-based medication used, for delta-9-tetrahydrocannabinol (THC), cannabidiol (CBD) and a combination of THC and CBD (THC:CBD). RESULTS: Thirty-eight studies were identified (THC = 23; CBD = 6; THC:CBD = 9; N = 2341, mean age: 63.
08 years, men: 53. 86%). THC had a very low incidence of all-cause and treatment-related adverse events (IR: 122. 18, 95% confidence interval [CI] 38. 23-253. 56; IR: 84. 76, 95% CI 0. 13-326. 01, respectively) and negligible serious adverse events (IR = 0). Similar IRs for CBD (all cause, IR: 111.
Buy Cbd: What Does The Science Say? - Page 259 - Google Books Result in NYC - limited time

24-495. 93; treatment related, IR: 1. 76, 95% CI 4. 63-23. 05) and no serious adverse events (IR = 0). CBD was not associated with a risk of treatment-related withdrawals. THC had a low risk of all-cause and treatment-related withdrawals (IR: 25. 18, 95% CI 12. 35-42. 52; IR: 7.
26-14. 38, respectively). The THC:CBD treatment had a low risk of all-cause and treatment-related adverse events (IR: 100. 72, 95% CI 0. 25-383. 00; IR: 55. 38, 95% CI 8. 61-142. 80, respectively), but reported a risk of all-cause and treatment-related serious adverse events (IR: 21. 32, 95% CI 0.
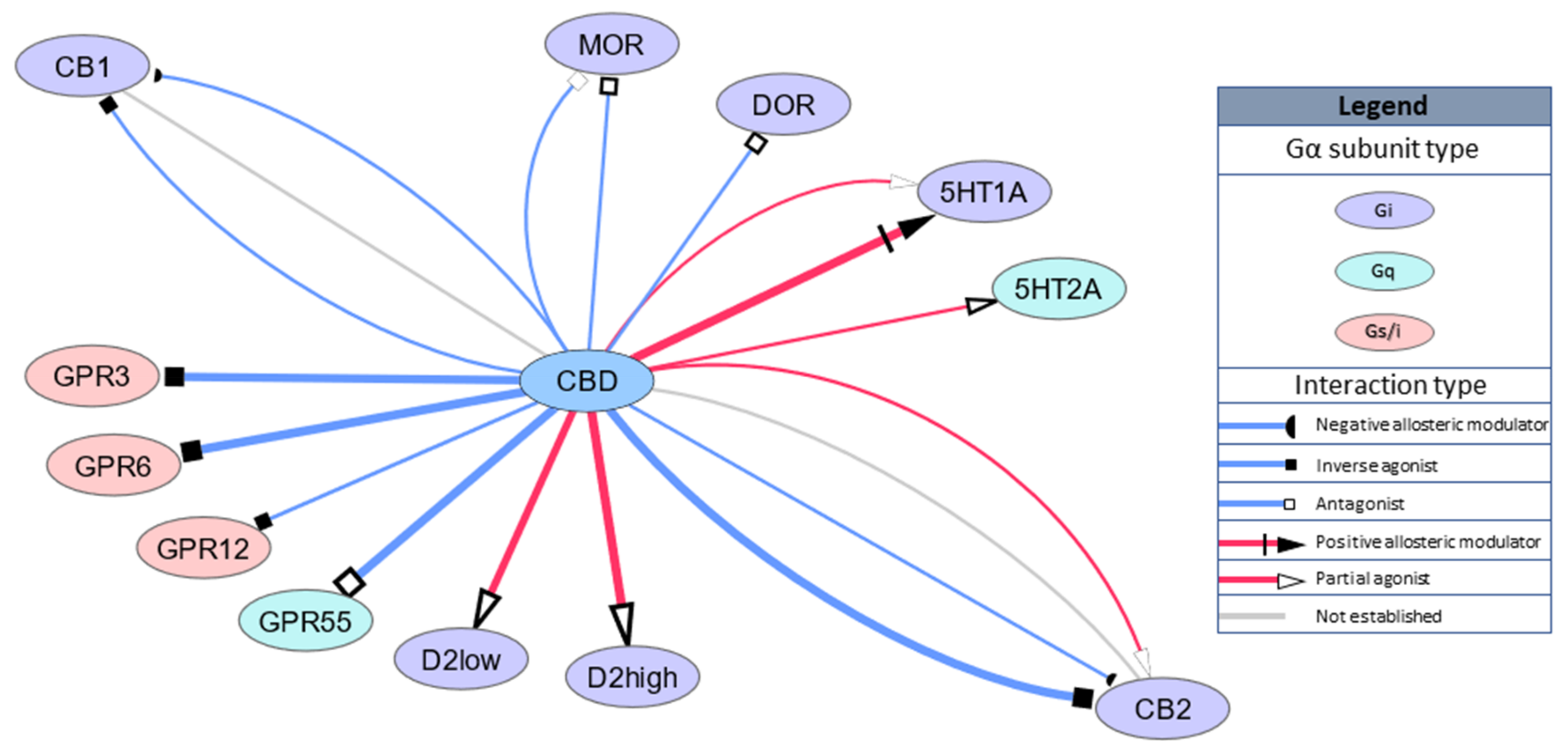
26; IR: 3. 71, 95% CI 0. 21-11. 56, respectively), and all-cause and treatment-related withdrawals (IR: 78. 63, 95% CI 17. 43-183. 90; IR: 34. 31, 95% CI 6. 09-85. 52, respectively). Significant heterogeneity (I2 >55%) was present in most analyses. CONCLUSIONS: Although cannabinoid-based medications were generally safe and acceptable to adults aged over 50 years, these estimates are limited by the lack of a control condition and considerable heterogeneity.
Therapeutic Efficacy Of Cbd - Aurora Cannabis now in LA
AB - BACKGROUND AND OBJECTIVE: Although cannabinoid-based medications are increasingly used by older adults, their safety and tolerability in this age group remain unclear. The purpose of this systematic review was to examine the safety and tolerability of cannabinoid-based medications by conducting a meta-analysis of open-label observational studies of cannabinoid-based medications for all indications in individuals with a mean age of ≥50 years.
Study quality was assessed using an adapted version of the Grading of Recommendations Assessment, Development and Evaluation criteria and Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines were followed. We included studies that (a) were published from 1990 onwards; (b) included older adults (mean age ≥50 years); and (c) provided data on the safety and tolerability of medical cannabinoids.
Risk of adverse events, serious adverse events and withdrawals was computed as the incidence rate (IR). Separate analyses were conducted by the cannabinoid-based medication used, for delta-9-tetrahydrocannabinol (THC), cannabidiol (CBD) and a combination of THC and CBD (THC:CBD). RESULTS: Thirty-eight studies were identified (THC = 23; CBD = 6; THC:CBD = 9; N = 2341, mean age: 63.
Table of Contents
Latest Posts
Buy 11 Most Accurate Heart Rate Monitors - Cityryde in NC - limited time only
The Best Heart Rate Monitors, Chest Straps And Watches now available in TEXAS - limited period
Buy Best Fitness Trackers 2023 - Live Science in NYC
More
Latest Posts
Buy 11 Most Accurate Heart Rate Monitors - Cityryde in NC - limited time only
The Best Heart Rate Monitors, Chest Straps And Watches now available in TEXAS - limited period
Buy Best Fitness Trackers 2023 - Live Science in NYC